Fast time to market is a key feature of our offering
Furthermore, we are addressing the dental industry’s need for agile and fast access to FDA cleared and CE approved dental implant markets.
We collaborate closely with your core technical personnel to articulate the vision and define the parameters of the final prosthetic solutions. This may include translating physics or process requirements into functional engineering specifications.
Avoid defects and variations
We specify requirements and areas of usage as early as possible – even in the conceptual and design phases – and every aspect of the development, from design to distribution, adheres to strict quality standards. All our activities have a common goal – to avoid any defects and variations, while ensuring documented traceability.
Our services within design and development also include regulatory services for product registrations and market approval. Additionally, we support our customers with development and technical documentation for privately labelled products.
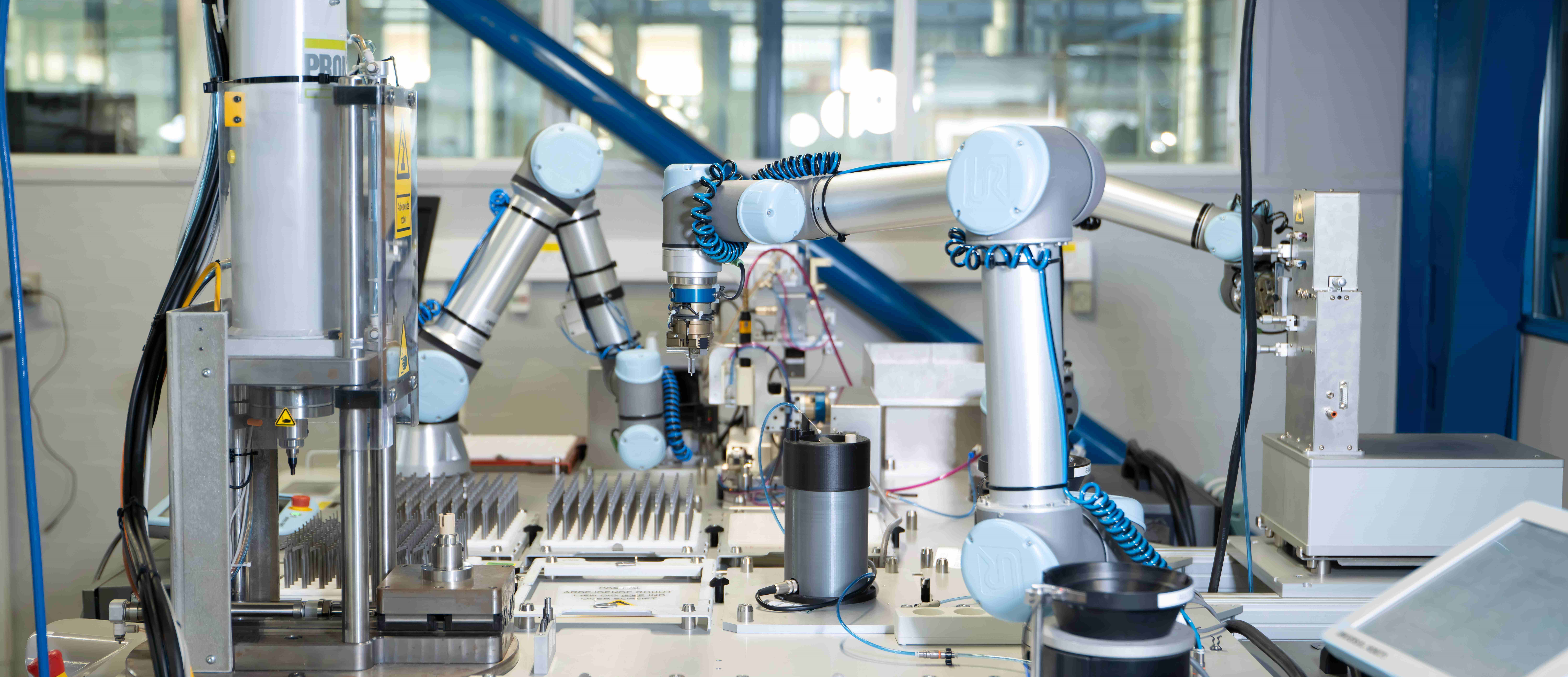
Co-creation of Your Products
The largest implant makers worldwide trust our expertise and have outsourced selected design, development, and manufacturing activities to us. Since we develop and manufacture our own branded dental components in the Elos Accurate® family, we have the experience you need to get to market fast.
With more than 50 years of experience as a solution partner for medical devices, we have extensive insights into how to design, develop, and manufacture medical devices according to applicable regulatory requirements.



