In-house. In control. In time.
Introducing the Elos Accurate® Pre-Milled Blank Solutions in U.S.
A complete solution
In-house milling of customized abutments saves time and produces abutments with demonstrated clinical and esthetic benefits. It also gives those performing the milling complete control over the process. All while meeting regulatory requirements thanks to our open, validated, FDA-compliant workflows.
The details that make it different
Blanks in titanium grade 5, a stainless-steel holder, a blanks library in 3shape and exocad, compatibility with all major implant systems—the Elos Accurate® Pre-Milled Blanks Solution is a comprehensive, ready-to-use system.
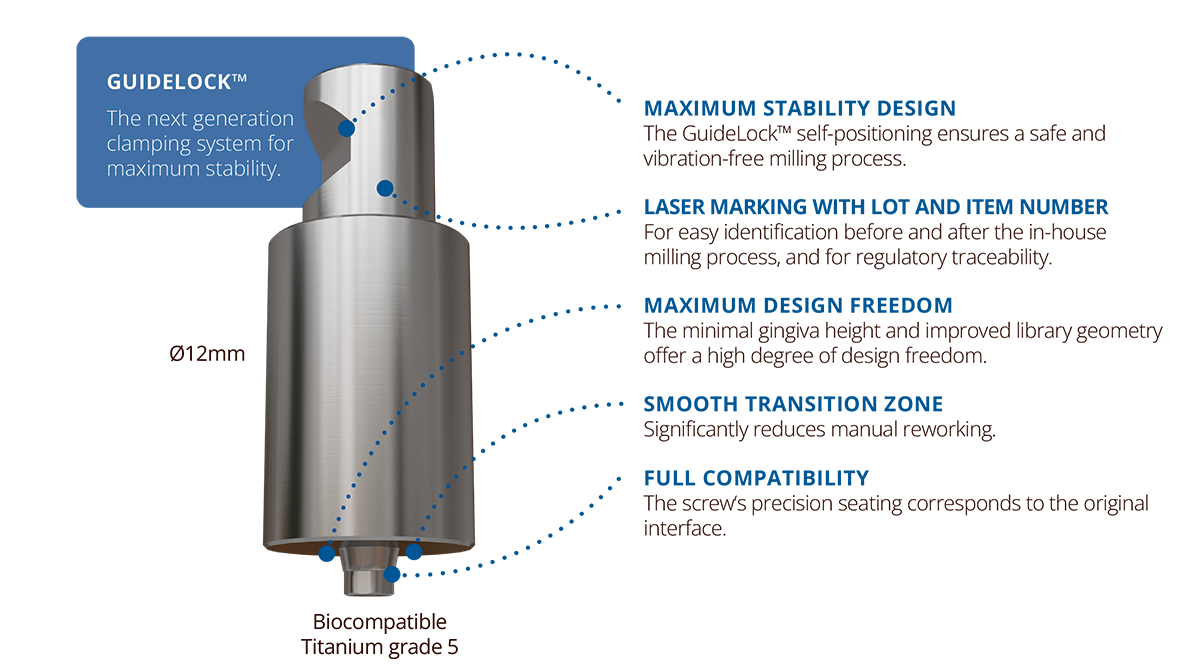
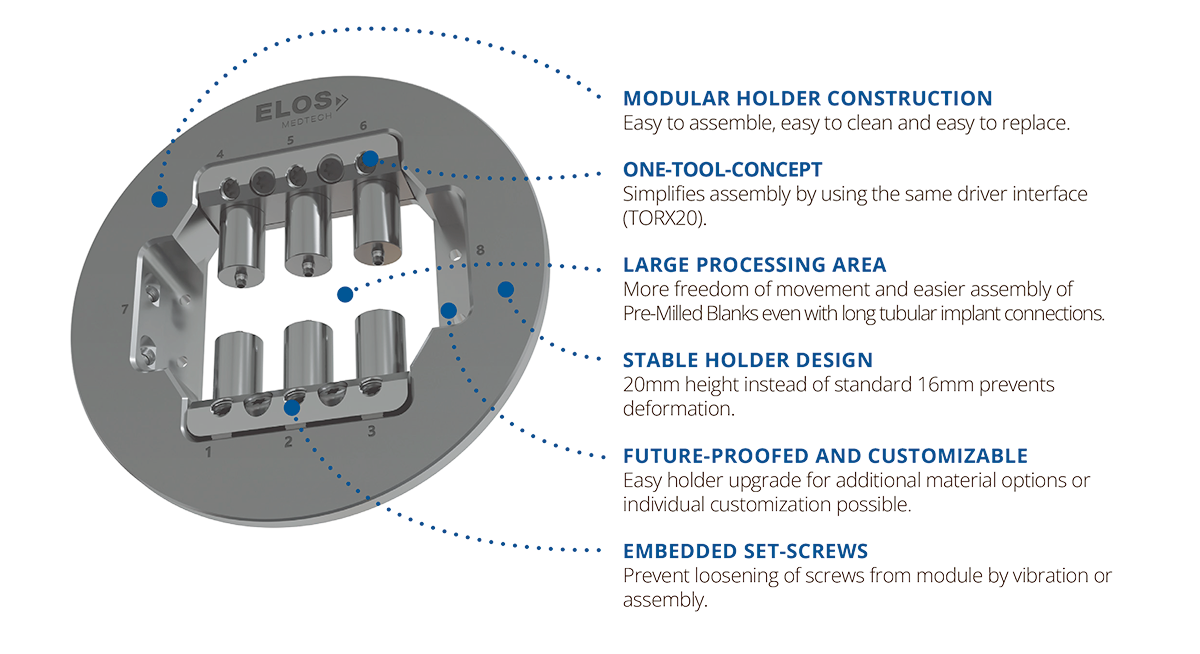
Maximize your milling throughput
The Elos holder—mandatory for milling Elos Pre-Milled blanks—lets you mill up to six different blanks in a single operation. Made from durable, high-quality stainless-steel, the holder is compatible with imes icore (One+, 150i, 250i, 350i and 650i), Planmeca PlanMill® 60S and vhf (R5, S5, N4+, Z4) milling machines.
Regulations and risk mitigation
In the US, the FDA classifies customer-specific milling as the manufacture of a Class II medical device. Labs milling custom implant abutments with CAD/CAM technology must
either
commission a validated milling center (VMS) with a listing of the facility and devices, and implement a Quality Management System
or
use products with a 510(k) pre-market clearance and registration with a validated digital workflow.

Solution
Using the FDA-cleared Elos validated workflow is essential for dental labs and clinics to produce customized abutments locally. It ensures compliance with regulatory requirements and helps produce restorations that meet the highest standards of safety and effectiveness, and will reduce the risk of liability issues for the lab.

